Amyloid Beta Aggregation
inhibition of β-amyloid aggregation by Cobalt(III) Schiff base complexes
Similar to the self-assembly of peptide amphiphiles, aggregation of amyloid β into fibrils found in brains of Alzheimer’s patients results from the intramolecular and intermolecular hydrogen bonding interactions that lead to formation of β-sheet networks. Alzheimer’s disease (AD) is a progressive, neurodegenerative disorder associated with memory loss and cognitive decline. Pathologically AD is characterized by aggregation of the amyloid beta (Aβ) peptide into extracellular amyloid plaques and the formation of intracellular tau tangles. The aggregation of the soluble peptide Aβ into oligomers and ultimately fibrils is widely believed to be the key pathogenic mechanism for neurotoxicity. In vivo, Aβ exists in two primary isoforms: Aβ40, which is more abundant (9:1 ratio in CSF), and Aβ42 which is far more prone to aggregation and thought to be more relevant to the disease process.
Coordination complexes have emerged as prominent modulators of amyloid aggregation via their interaction with the N-terminal histidine residues of amyloid-β. Here, we reported the synthesis and characterization of a novel cobalt(III) Schiff base complex with methylamine axial ligands, and presented both computational and experimental data demonstrating the reduction of β-sheet formation by this complex.
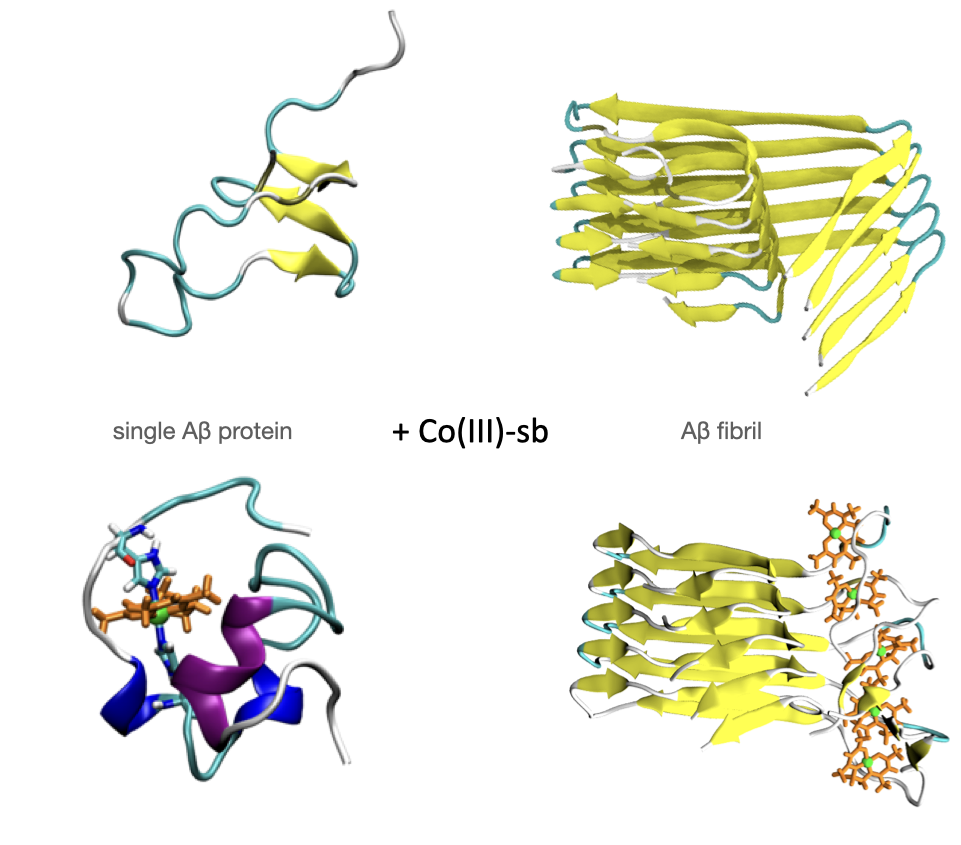
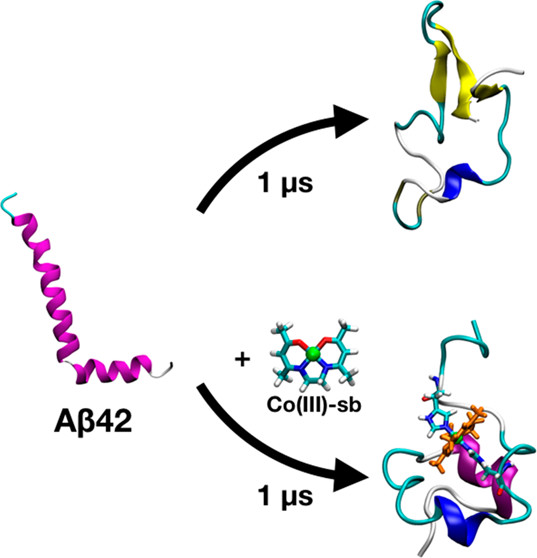
Using atomistic computational models and experiments, we have shown the efficiency of Co(III)-sb in inhibiting Aβ aggregation as a potential therapeutic for Alzheimer’s disease. MD simulations of bound Co(III)-sb on monomeric Aβ folding demonstrated reduction in β-sheet formation, decreased intermolecular hydrogen bonding between the N- and C-termini, and increased distance between residues. These effects are primarily due to the placement of Co(III)-sb bound bidentate between His6 and His13. The strong bond between the histidine residues and cobalt prevents Aβ42 from freely interacting with itself and water. Co(III)-sb sterically hinders the Aβ42 side chains from moving closer and making hydrogen bonds that would eventually result in β-sheets. The simulations of pentameric Aβ42 oligomers demonstrate the ability of Co(III)-sb to destabilize the hydrogen bonding required for amyloid aggregation. Furthermore, our newly synthesized Co(III)-sb complex (Co(MeNH2)2 improved inhibition of aggregation compared to unmetalated acacen ligand as well as untreated Aβ. With this combined computational and experimental approach, we hope to provide insights on interactions of Co(III)-sb with Aβ that can be used for the effective development of therapeutics for Alzheimer’s disease.
References
2019
-
 Inhibition of Amyloid-βAggregation by Cobalt(III) Schiff Base Complexes: A Computational and Experimental ApproachJournal of the American Chemical Society, Oct 2019
Inhibition of Amyloid-βAggregation by Cobalt(III) Schiff Base Complexes: A Computational and Experimental ApproachJournal of the American Chemical Society, Oct 2019